Chemical Profile and Molecular Structure of Proteins in Air Dried and Ensiled Watermelon Rind (Citrullus Lanatus)
Keywords:
Molecular Spectral, Protein, Nutrient Variation, Polypeptide, Chemical Profile.Abstract
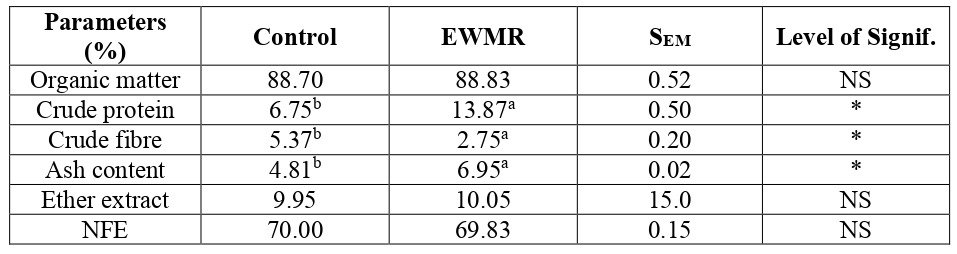
The structure of protein in feedstuffs determines their quality and rate of responsiveness to digestible enzymes and utilization. This study was to investigate the magnitude of the differences in protein structure at the molecular level as well as the nutritive value of dried (control) and ensiled watermelon rind for use as protein source in livestock diets. Protein chemical profile and both primary and secondary molecular structure of dried (control) and ensiled watermelon rind samples were determined by standard procedures of AOAC and FTIR molecular spectroscopy, respectively. The results showed significant (P<0.05) differences in chemical profile parameters of crude protein and crude fibre as ensiled samples recorded higher crude protein (13.8%) than control (6.72%). Dry matter content however, recorded similar values. Results from protein molecular structure, showed that ensiling significantly (P<0.05) increased the spectral intensity of primary protein features of amide I area, amide II area, amide I height, amide II height and Protein secondary structure of α-helix and β-sheet height compared to control. The ratio of α-helix to β-sheet height decreased significantly (P<0.05) when compared to the control. Vibrational molecular spectroscopy could therefore, be used to detect inherent structural protein make-up characteristics of feedstuffs toward predicting protein quality of feeds.
Downloads
Published
How to Cite
Issue
Section
Copyright (c) 2024 Authors

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.




